METHODS AND ANALYSES
Developing State of the Art Analytical Methods
Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS) is a powerful analytical technique widely used in geochemistry for in situ analysis of solid materials. It combines a laser ablation system to sample the solid material with an ICP-MS for precise elemental and isotopic measurements.
We develop and implement a broad spectrum of LA-ICP-MS methods to analyze trace elements and isotopic systems, including U-Pb geochronology, Sr, Nd, Hf, B, and S isotopes, tailored to address diverse geological and environmental questions. Our expertise encompasses high-precision U-Pb dating to understand the timing of geological events, as well as Sr and Nd isotopes for tracing fluids and crustal evolution. For example, we utilize Hf isotopes in zircon for insights into mantle-crust interactions and magma differentiation, while B isotopes are applied to paleoenvironmental reconstructions and fluid interactions. Sulfur isotopes further enhance our understanding of redox processes in ore deposits and environmental systems. These methodologies are all via in situ, high-spacial resolution analysis, and are ideal for examining the chemical composition and isotopic signatures of minerals, fossils, and geological materials, providing critical insights into Earth’s history and processes.
We also specialize in developing reference materials tailored for LA-ICP-MS applications, which are essential for achieving precise and accurate isotope ratio measurements. These reference materials must be rigorously characterized at the micro-scale as they are vital for calibrating instruments and validating results across a range of geological samples. The choice of reference material depends heavily on the isotopic system and matrix under investigation, requiring close attention to matrix matching to minimize matrix effects that could impact isotopic fractionation during analysis. By developing or carefully selecting matrix-matched reference materials, we enhance the precision and reliability of LA-ICP-MS measurements, enabling robust isotopic and trace element analyses. Click Here for more details
THE PRINCIPLE
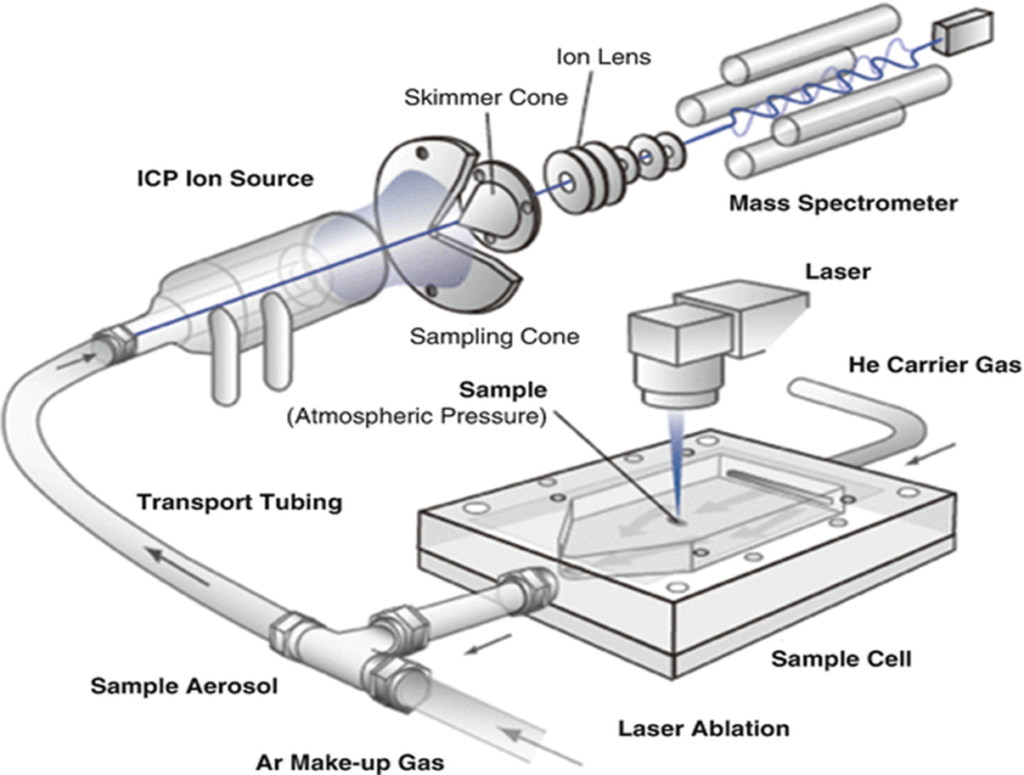
Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS) is a technique used for precise analysis of the elemental and isotopic composition of solid samples. The process begins with the sample placed inside a sealed chamber, called the sample cell. A focused laser beam is directed onto the sample’s surface, creating small craters as it ablates or vaporizes tiny amounts of material. This ablated material forms an aerosol, which is carried out of the cell by a flow of helium gas. The aerosol then mixes with argon gas (Ar) in transport tubing, optimizing the transport conditions for the particles and ions.
The mixed gas containing the sample aerosol is introduced into the Inductively Coupled Plasma (ICP) ion source. Here, the plasma, a high-temperature ionized gas, further breaks down the sample material into individual ions. These ions are drawn through a series of cones, including a sampling cone and a skimmer cone, before being focused into a beam by an ion lens. The ion beam then enters the mass spectrometer, where the ions are separated based on their mass-to-charge ratio (m/z). This allows for the detection and quantification of elements or isotopes within the sample with high precision and sensitivity, making LA-ICP-MS a powerful tool for geochemical, environmental, and material sciences.
- Advantages: The technique provides high spatial resolution, enabling analysis of small areas within samples, making it ideal for heterogeneous materials like minerals, fossils, and other geological specimens. It also offers low detection limits and high sensitivity for trace elements and isotopes.
1. Trace Element Analysis
- Capabilities: LA-ICP-MS is particularly effective for analyzing trace elements (ppm to ppb levels) in geological materials, such as zircon, apatite, calcite, and feldspar.
- Applications:
- Geochemical Fingerprinting: Trace element compositions can distinguish between different rock types and provide information on the petrogenesis of magmas.
- Provenance Studies: Trace elements in detrital minerals like zircon can trace sediment sources and sedimentary basin evolution.
- Paleoenvironmental Reconstruction: Elements like Sr, Mg, and Ba in carbonate minerals can be used to infer past ocean temperatures, salinity, and other environmental conditions.
- Ore Deposit Studies: Characterizing trace elements in minerals helps in understanding mineralization processes and the formation of economic ore deposits.
2. U-Pb Geochronology
- Methodology: LA-ICP-MS is widely used for U-Pb dating, especially for minerals like zircon, monazite, titanite, and apatite. U and Pb isotopic ratios are measured to determine the age of crystallization or metamorphism.
- We currently use the Element 2 Sector Field ICP MS for most U-Pb geochronology. The Agillent 8800 triple quadrupole is also available for U-Pb analyses. All the Laser Ablation is done in a RESOLution 193 nm Laser.
- Applications:
- Dating of Igneous and Metamorphic Rocks: U-Pb dating is fundamental in constraining the ages of igneous intrusions, metamorphic events, and magmatic crystallization.
- Detrital Zircon Studies: U-Pb dating of detrital zircons provides insights into sedimentary provenance, crustal evolution, and tectonic history.
- Paleoenvironmental Studies: U-Pb ages in fossils and carbonate rocks, such as Orthoceras, help understand the timing of biotic and environmental events in Earth’s history.
3. Strontium (Sr) Isotopes
- Isotopes: The most common ratio used in geology is ⁸⁷Sr/⁸⁶Sr, which evolves due to the decay of ⁸⁷Rb.
- We use the Neoma ICP MS for most U-Pb geochronology. The Agillent 8800 triple quadrupole is also available for U-Pb analyses. All the Laser Ablation is done in a RESOLution 193 nm Laser.
- Applications:
- Provenance Studies: Variations in ⁸⁷Sr/⁸⁶Sr ratios in sediments and fossils can trace the sources of sediments or water bodies and reconstruct past hydrological conditions.
- Diagenesis and Alteration Studies: Sr isotopes are used to assess post-depositional changes in rocks and fossils, providing insights into the diagenetic history of carbonate rocks.
- Environmental Reconstructions: Sr isotopes in biogenic carbonates like corals and shells reflect past seawater composition and help in reconstructing marine conditions.
4. Neodymium (Nd) Isotopes
- Isotopes: The ratio ¹⁴³Nd/¹⁴⁴Nd is used to study mantle differentiation and crustal evolution.
- We use the Neoma ICP MS for most U-Pb geochronology. The Agillent 8800 triple quadrupole is also available for U-Pb analyses.
- Applications:
- Crustal Evolution: Nd isotopes in rocks and minerals are used to track the formation and evolution of continental crust through time, providing insights into tectonic processes.
- Provenance Studies: Nd isotopes in sedimentary rocks and minerals help identify the sources of sediments, aiding in reconstructing paleo-drainage systems.
- Petrogenetic Studies: Nd isotopic compositions are key to understanding the formation and evolution of igneous and metamorphic rocks.
5. Hafnium (Hf) Isotopes
- Isotopes: The ¹⁷⁶Hf/¹⁷⁷Hf ratio in zircon is commonly used for isotopic studies.
- We use the Neoma ICP MS for most U-Pb geochronology. The Agillent 8800 triple quadrupole is also available for U-Pb analyses.
- Applications:
- Crust-Mantle Differentiation: Hf isotopes provide insights into the timing and processes of crust formation and recycling through interactions between the mantle and crust.
- Detrital Zircon Provenance: Hf isotopic compositions in detrital zircons complement U-Pb dating to provide information on the original source rock’s evolution.
- Magmatic Evolution: Hf isotopes in zircon can reveal magma sources, processes of contamination, and differentiation.
6. Boron (B) Isotopes
- Isotopes: Boron isotopic ratios (¹¹B/¹⁰B) are sensitive to variations in fluid compositions.
- Applications:
- Geothermal Systems: B isotopes are used to track fluid sources and interactions in geothermal and hydrothermal systems.
- Paleoceanography: B isotopes in marine carbonates are employed to reconstruct past ocean pH and CO₂ levels, providing insights into climate change.
- Subduction Zone Processes: Variations in B isotopic ratios in volcanic rocks provide evidence of subduction-related processes and slab-fluid interactions.
7. Sulfur (S) Isotopes
- Isotopes: S isotopes (³⁴S/³²S) are critical for understanding redox processes and biogeochemical cycles.
- We use the Neoma ICP MS for most U-Pb geochronology. The Agillent 8800 triple quadrupole is also available for U-Pb analyses.
- Applications:
- Ore Deposit Studies: S isotopes in sulfide minerals help in understanding ore-forming processes and fluid sources, aiding in the exploration of mineral deposits.
- Environmental Geochemistry: S isotopes are used to trace anthropogenic pollution sources and natural biogeochemical cycles in water systems.
- Paleoenvironmental Reconstruction: S isotopic studies in carbonate-associated sulfate and pyrite provide information on ancient ocean chemistry and redox conditions.
8. Rb-Sr Dating
Isotopes: The ⁸⁷Sr/⁸⁶Sr ratio is a key metric in geological studies, evolving over time due to the radioactive decay of ⁸⁷Rb into ⁸⁷Sr. This decay process allows for the study of isotopic variations in rocks and minerals, which can reveal significant geological events and processes.
We utilize the Neoma ICP-MS for precise Sr isotope analysis, and the Agilent 8800 triple quadrupole ICP-MS is available for complementary analyses. All our laser ablation work, including Sr isotopic studies, is performed using a RESOlution 193 nm excimer laser, ensuring high spatial resolution and accuracy.
The method uses the decay of ⁸⁷Rb to ⁸⁷Sr, with a half-life of approximately 48.8 billion years, to date geological materials. The technique measures the ⁸⁷Rb/⁸⁷Sr and ⁸⁶Sr ratios to create an isochron plot, where the slope indicates the age of the sample and the intercept gives the initial ⁸⁷Sr/⁸⁶Sr ratio. Rb-Sr dating is particularly useful for determining the ages of igneous and metamorphic rocks and reconstructing the thermal histories of geological terrains. It is also effective in studying sedimentary processes when minerals like micas, feldspars, or specific carbonates are present. Accuracy in Rb-Sr dating depends on the sample’s ability to remain a closed system, with no gain or loss of Rb or Sr since formation, ensuring reliable age determinations.
General Applications of LA-ICP-MS in Earth Sciences
- Environmental Studies: Trace element and isotopic analysis of carbonates, bones, and other materials help reconstruct past environmental changes, including pollution history and paleoceanography.
- Geochronology and Tectonics: U-Pb, Sr, Nd, and Hf isotopes are critical for understanding the timing of geological events, from the formation of continental crust to the evolution of tectonic regimes.
- Isotope Systematics in Fossils: Analyses of Sr, B, and U-Pb isotopes in fossils provide insights into past seawater chemistry, diagenetic processes, and geological time scales.
- Petrogenesis and Metamorphism: LA-ICP-MS allows detailed study of mineral compositions, aiding in understanding the processes of magma genesis, metamorphism, and crustal evolution.
LA-ICP-MS, with its versatility and precision, has transformed how we study the Earth’s history, providing detailed insights into the processes that shape our planet. Its application ranges fr
