ELECTRON MICROPROBE
An electron microprobe, also known as an electron probe microanalyzer (EPMA), is a high-precision instrument used to determine the chemical composition of small areas of solid materials at the micro to nanoscale. It works by directing a focused beam of electrons onto the sample, which causes the emission of characteristic X-rays from the elements within the sample. These X-rays are then analyzed to identify and quantify the elemental composition with high accuracy. Unlike SEMs, which focus mainly on imaging, electron microprobes are specifically designed for detailed chemical analysis, making them essential tools in fields like geology, materials science, and metallurgy for analyzing mineral phases, metals, and other solid materials.
________________________________________________________________________________________________________________
Download the presentation here:
++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++

How an Electron Microprobe (EPMA) Works:
- Electron Beam Generation:
- Like an SEM, the electron microprobe generates a focused beam of high-energy electrons (typically in the range of 5-30 keV) using an electron gun. The electron beam is directed onto the sample, exciting the atoms within the material.
- X-ray Emission:
- When the high-energy electron beam interacts with the atoms in the sample, it displaces electrons from their inner atomic shells. This displacement causes the atom to be in an excited state.
- To return to a lower energy state, electrons from outer shells drop into the inner shell vacancies, releasing characteristic X-ray photons. These X-rays are unique to each element and can be used to identify and quantify the elements present in the sample.
- Wavelength Dispersive Spectrometry (WDS):
- The X-rays emitted from the sample are analyzed using wavelength dispersive spectrometers (WDS). WDS uses a crystal to diffract the X-rays according to their wavelength (following Bragg’s Law), allowing for high-resolution separation of the X-rays from different elements.
- This makes WDS highly sensitive, allowing it to distinguish between elements with similar X-ray energies and achieve high quantitative precision.
- Quantitative Analysis:
- By measuring the intensity of the X-rays for specific elements, EPMA can determine the concentration of elements in the sample with high accuracy, often down to parts per million (ppm).
- The instrument is typically calibrated using known standards, and sophisticated software is used to correct for various effects, such as X-ray absorption and fluorescence.
- Secondary and Backscattered Electron Imaging:
- Like an SEM, the electron microprobe can also produce high-resolution images of the sample’s surface using secondary electrons (for topographic imaging) and backscattered electrons (for compositional contrast). This allows researchers to correlate the chemical data with the sample’s microstructure.

Key Features of an Electron Microprobe (EPMA):
- Quantitative Elemental Analysis:
- EPMA is capable of performing precise quantitative chemical analyses. It can measure the concentrations of major, minor, and trace elements in a sample with high accuracy, typically within 1-2% for major elements and down to ppm levels for trace elements.
- Spatial Resolution:
- The electron beam can be focused to very small diameters (on the order of 1 micron or less), allowing for the analysis of very small features in a sample. This high spatial resolution makes EPMA particularly useful for studying individual mineral grains, inclusions, or phases in geological samples.
- Wavelength Dispersive Spectrometry (WDS):
- WDS provides excellent spectral resolution, allowing for the detection and differentiation of elements that may have overlapping X-ray emission lines. This makes EPMA more precise than energy-dispersive X-ray spectroscopy (EDS/EDX) systems typically found in SEMs.
- Non-Destructive Analysis:
- EPMA is largely non-destructive, meaning that samples can be analyzed without being significantly altered or damaged. This is important for rare or valuable materials.
- Versatile Sample Types:
- EPMA can be used on a wide range of solid samples, including rocks, minerals, metals, ceramics, and glasses. The only requirement is that the sample needs to be stable under the high vacuum required for operation.
Sample Preparation for EPMA:
- Polished Thin Sections:
- Samples must typically be polished to a smooth, flat surface to ensure accurate X-ray analysis and imaging. In geological applications, samples are often prepared as polished thin sections mounted on glass slides.
- Conductive Coating:
- Non-conductive samples (e.g., geological specimens, ceramics) are usually coated with a thin layer of carbon or gold to prevent charging effects under the electron beam and to ensure stable imaging and analysis.
Advantages of EPMA:
- High Precision and Accuracy:
- EPMA is renowned for its ability to measure elemental concentrations with high precision and accuracy, making it a key tool in many fields for detailed chemical analysis.
- Spatially Resolved Analysis:
- EPMA allows for analysis at the micron scale, enabling researchers to examine the chemical composition of individual phases, grains, or inclusions within a heterogeneous sample.
- WDS Sensitivity:
- The WDS system used in EPMA provides better spectral resolution than EDS, reducing peak overlap and improving the detection of trace elements.
- Elemental Mapping:
- EPMA can produce detailed elemental maps showing the distribution of elements across the sample surface. This is valuable in identifying compositional zoning, inclusions, or chemical heterogeneities in materials.
- Phase Analysis:
- EPMA is useful for characterizing different phases within a material, such as distinguishing between different minerals in rocks or identifying phases in alloys.
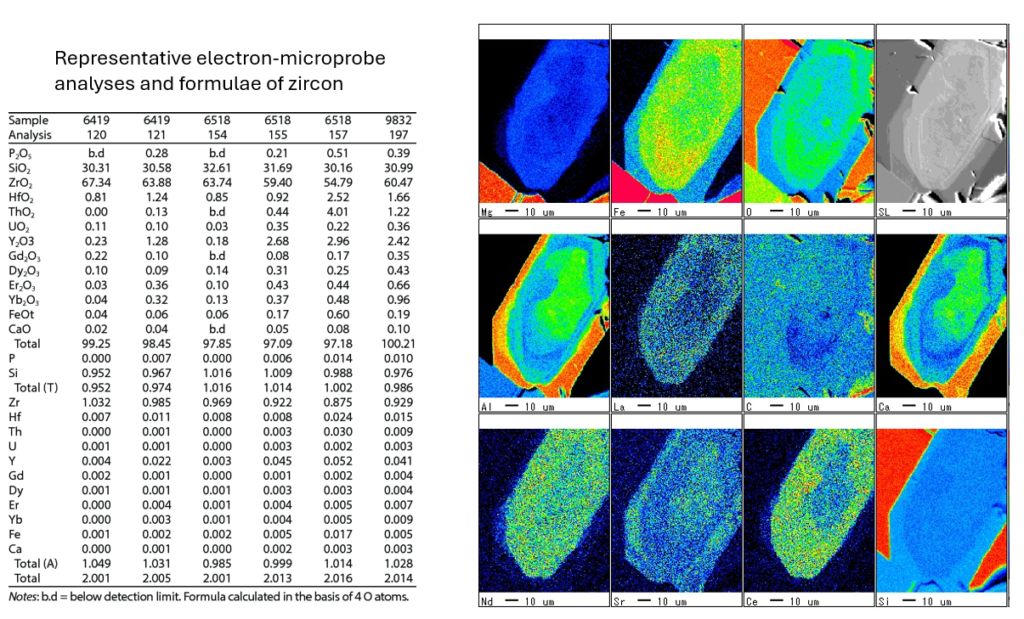
On the right, the accompanying images are element distribution maps from a zircon grain, showing the spatial concentration of elements such as Mg, Fe, O, Al, La, Sr, and others. These maps illustrate how different elements are distributed across the grain, helping identify zoning patterns, growth histories, and possible alterations. The scale bar indicates 10 µm, providing a sense of the spatial resolution. Together, the table and images provide detailed chemical and spatial information, crucial for understanding the zircon’s geochemical history and conditions during crystallization.
Limitations of EPMA:
- Longer Analysis Time:
- Because of the high precision required for WDS, EPMA analysis can be slower than EDS. Quantitative analysis of multiple elements may take several minutes per spot, and full elemental mapping can take even longer.
- Vacuum Requirement:
- Like SEM, EPMA operates under a high vacuum, limiting its ability to analyze volatile or hydrated samples. Biological and other delicate materials need special preparation before analysis.
- Surface Sensitivity:
- EPMA primarily probes the surface or near-surface region of the sample. Deeply buried features cannot be analyzed directly, limiting the technique to surface or near-surface studies.
- Conductive Coating:
- Non-conductive samples must be coated with a conductive material, which can mask or interfere with the detection of light elements like carbon or oxygen.
Applications of EPMA:
- Geology and Mineralogy:
- EPMA is widely used for determining the chemical composition of minerals in rocks, particularly in petrology and geochemistry. It is essential for studying mineral phases, zoning patterns, and inclusions in rocks, and for determining the provenance of geological materials.
- Materials Science and Metallurgy:
- EPMA is used to analyze the chemical composition of metals, alloys, ceramics, and other materials, providing insights into their phase composition, impurities, and performance characteristics.
- Environmental Science:
- EPMA can be used to analyze pollutants and contaminants in environmental samples, such as soil or sediment, to track heavy metal contamination or other toxic elements.
- Archaeology and Art Conservation:
- EPMA helps in analyzing ancient artifacts and artworks to understand the materials used in their production, aiding in conservation efforts or providing insights into historical manufacturing techniques.
- Semiconductor Industry:
- In semiconductor fabrication, EPMA is used to analyze the composition of thin films, layers, and features on microchips, ensuring that materials meet strict chemical specifications.
Summary:
The Electron Microprobe (EPMA) is a highly sophisticated tool for quantitative elemental analysis at the microscopic scale. It combines the high-resolution imaging capabilities of an SEM with the precise chemical analysis provided by Wavelength Dispersive Spectrometry (WDS). EPMA is indispensable in fields like geology, materials science, and metallurgy for its ability to accurately measure the composition of complex, heterogeneous materials with micron-level spatial resolution. While it has limitations in speed and sample preparation, its accuracy and precision make it one of the most reliable techniques for elemental microanalysis.
